This whitepaper provides an in-depth analysis of the patent landscape in fields of MASH (Metabolic-Associated Steatohepatitis) biomarkers and Point of Care (PoC) kits. By examining key patent applications and identifying the leading players in the field, this paper offers valuable insights into the technological advancements, competitive dynamics, and potential opportunities within this space.
Introduction
The fields of MASH (Metabolic-Associated Steatohepatitis) biomarkers and Point of Care (PoC) kits for MASH diagnostics are experiencing rapid growth and innovation. This surge is driven by the recent Rezdiffra approval and the increasing demand from physicians to detect and monitor treatment responses to the first approved MASH drug. This growth is further reflected in the consistent patenting activity, signifying intense research and development efforts by biotechnology and pharmaceutical companies, academic institutions, and healthcare technology firms.
The Kits & PoC AIML domain focuses on developing point-of-care testing devices and artificial intelligence-based monitoring systems. These technologies aim to revolutionize patient care by enabling rapid on-site diagnosis and real-time health condition monitoring. By integrating cutting-edge AI and machine learning algorithms, these innovative solutions have the potential to enhance the accuracy, efficiency, and accessibility of diagnostic processes.
Conversely, the Biomarkers MASH domain centers on discovering and validating novel biomarkers specific to MASH. MASH, a severe form of metabolic-associated fatty liver disease (MASLD), is characterized by liver inflammation, cell damage, and progressive fibrosis, which can lead to cirrhosis and liver failure if untreated. Developing reliable non-invasive biomarker tests is crucial for early detection and monitoring of MASH progression, enabling timely intervention and personalized management strategies.
This whitepaper provides an in-depth analysis of the patent landscape in these two pivotal domains. By examining key patent applications and identifying the leading players in the field, this paper offers valuable insights into the technological advancements, competitive dynamics, and potential opportunities within the Kits & PoC AIML and Biomarkers MASH spaces.
Moreover, the whitepaper presents strategic insights for competitors seeking to establish a strong presence in these domains. It highlights the importance of focusing on innovation, collaboration, and IP protection to gain a competitive edge. By leveraging the findings of this patent landscape analysis, companies can make informed decisions regarding their research and development efforts, partnership strategies, and market positioning.
As the demand for advanced diagnostic and monitoring solutions continues to grow, the Kits & PoC AIML and Biomarkers MASH domains are poised for significant expansion. This whitepaper serves as a valuable resource for stakeholders looking to navigate the complex and rapidly evolving landscape of these fields, offering guidance on capitalizing on the opportunities presented by these transformative technologies.
Overview of the Space
Several emerging areas for new patenting opportunities are available in the Biomarkers MASH and Kits & PoC AIML spaces. These new patenting spaces represent potential avenues for innovation and intellectual property protection, helping companies and researchers establish a competitive edge in these rapidly evolving fields.
Overview of the New Patenting Space in Biomarkers MASH:
- Novel MASH Biomarkers: The discovery and validation of new biomarkers specific to MASH could lead to the development of innovative diagnostic tools, creating opportunities for patent protection in this space.
- Non-Invasive Diagnostic Methods: Advancements in non-invasive diagnostic techniques, such as liquid biopsies or advanced imaging technologies, could provide alternatives to liver biopsy for MASH diagnosis, presenting new patenting opportunities.
- Multi-Biomarker Diagnostic Panels: Developing diagnostic tools that utilize a combination of biomarkers rather than a single marker could improve the accuracy and specificity of MASH detection and monitoring, opening up new possibilities for patent protection.
- Predictive MASH Biomarkers: Identifying biomarkers that can predict disease progression or treatment response could enable more personalized management strategies for MASH patients, creating new patenting spaces for precision medicine approaches.
Overview of the New Patenting Space in Kits & PoC AIML:
- Wearable and Implantable Sensors: Integrating novel sensing technologies, such as wearables or implantable sensors, with point-of-care testing devices could enable continuous real-time monitoring of patient health, opening up new possibilities for patent protection.
- Advanced AI Algorithms: Developing cutting-edge AI and machine learning algorithms specifically designed for point-of-care data analysis and interpretation could lead to more accurate and efficient diagnostic tools, presenting opportunities for new patents.
- Miniaturized and Portable Devices: Innovations in device miniaturization and portability aimed at expanding the accessibility of point-of-care testing could create new patenting spaces for compact, user-friendly diagnostic tools.
- Multiplexed Testing Platforms: Developing point-of-care devices capable of performing multiple tests simultaneously could streamline diagnostic processes and provide a more comprehensive assessment of a patient’s health, opening up new avenues for patent protection.
Understanding the existing patent landscape offers insights into new patenting spaces not already occupied by existing intellectual property rights. Companies and researchers that understand their IP landscapes can strategically focus their R&D and product development efforts and position themselves to capitalize on emerging opportunities in the Biomarkers MASH and Kits & PoC AIML technologies market.
Patenting Activity
The patenting activity in the fields of Biomarkers MASH and Kits & PoC AIML shows a robust pattern of filings over recent years. The data indicates:
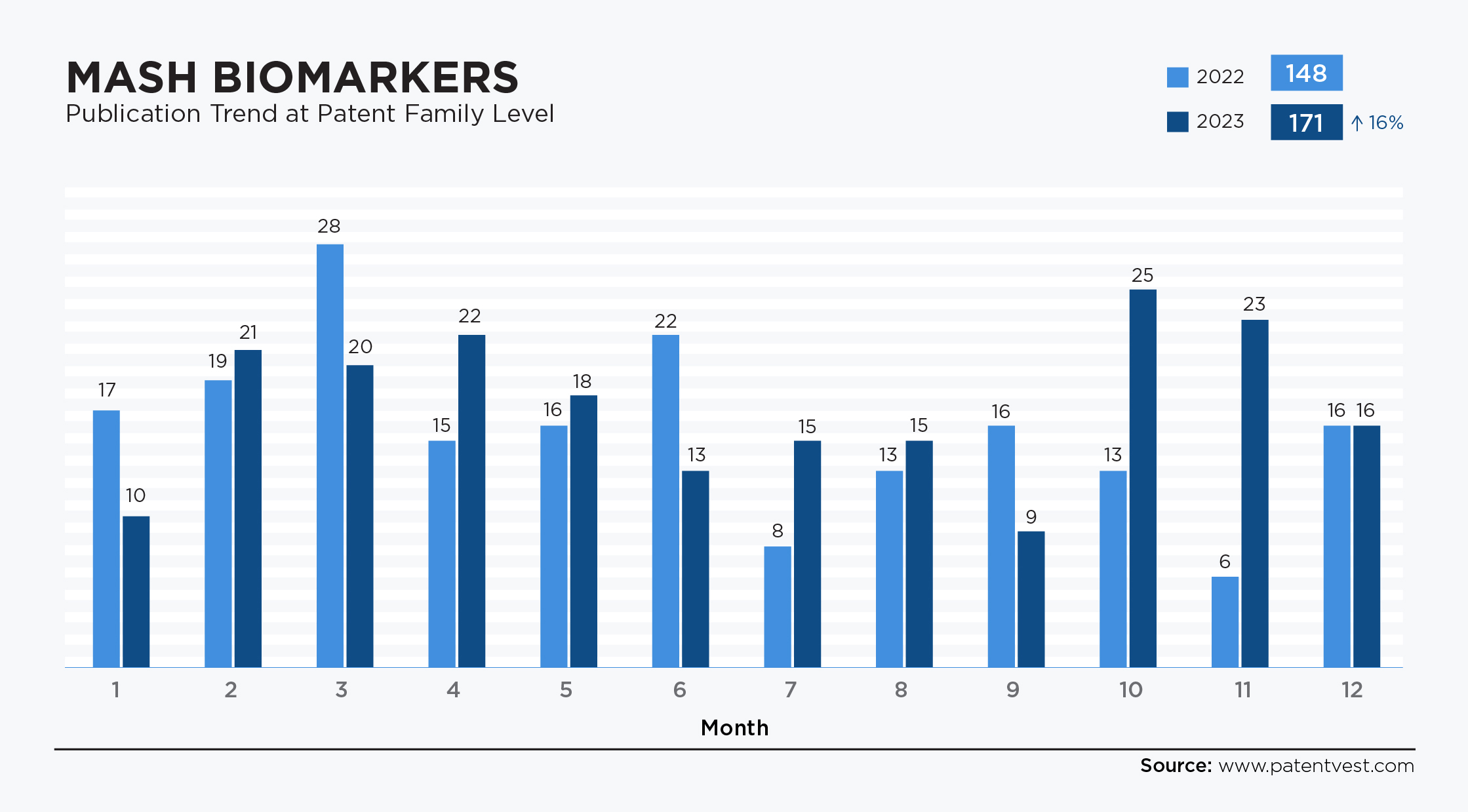
- Biomarkers MASH: The sector has seen a significant increase in patent filings. In 2022 and 2023, filings rose 16%, from 148 patent families to 171, particularly in areas related to the identification and validation of new biomarkers for the early detection, diagnosis, and monitoring of MASH.
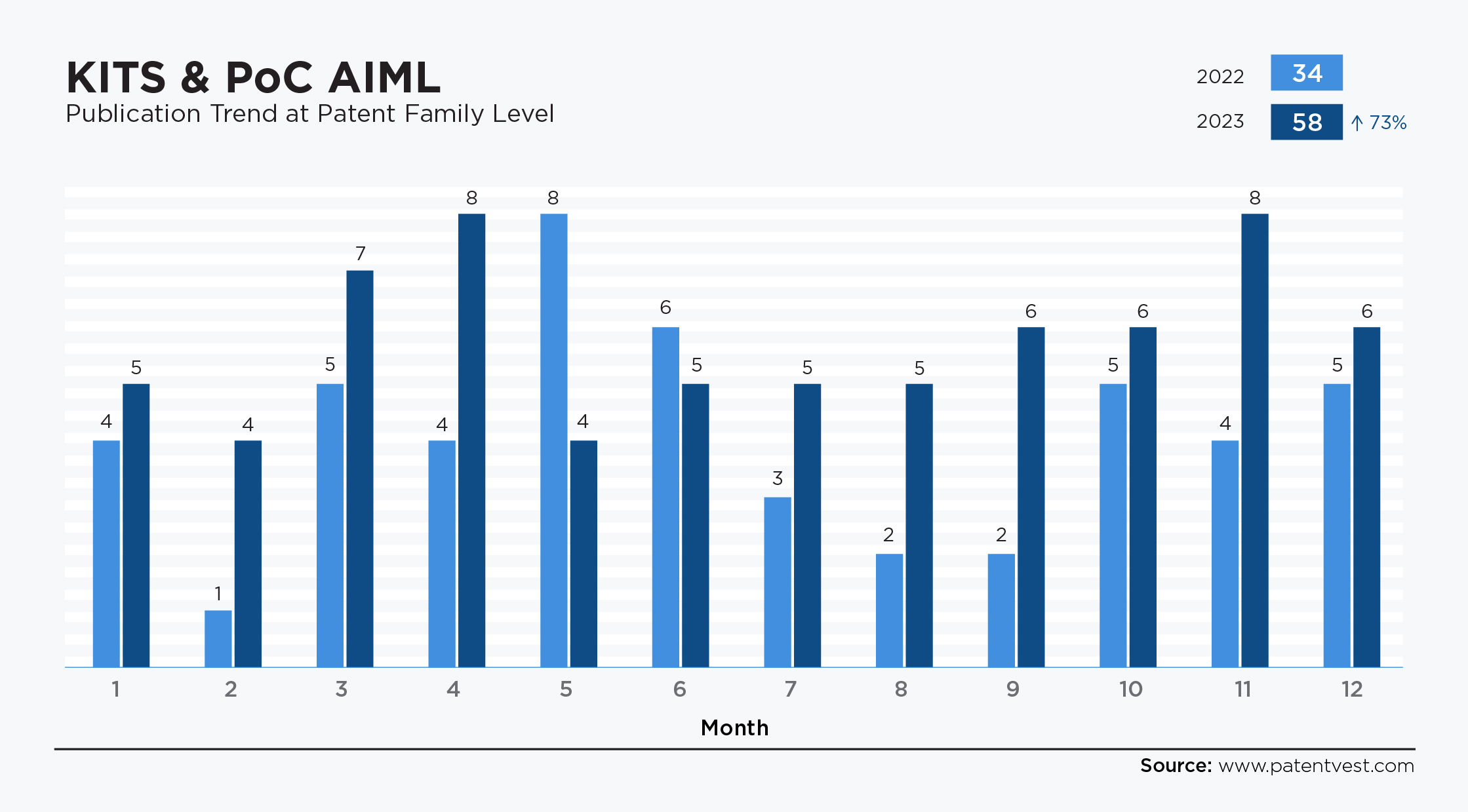
- Kits & PoC AIML in 2024: Numerous patents have been filed. In 2022 and 2023, filings rose 73%, from 33 patent families to 57, particularly in areas related to applications focusing on integrating AI technologies with point-of-care diagnostic tools.
These details highlight the ongoing innovation and strategic importance of intellectual property in these healthcare sectors, signaling continued growth and competitive developments.
Key Patent Applications
Several notable patent applications have been published in these spaces, highlighting the innovative work being done by various companies and research institutions. Some identified key patent applications along with their assignees in the fields of Biomarkers MASH and Kits & PoC AIML are as follows:
Biomarkers MASH:
- Significant Technology Advancement:
- Identification of novel biomarkers specific to MASH, such as genetic markers and metabolic signatures.
- Development of non-invasive diagnostic tools based on MASH biomarkers, such as blood tests and imaging techniques.
- Biomarker-based monitoring systems for assessing disease progression and treatment response.
- Key Patents:
- CN117538536A: Describes a novel biomarker for diagnosing MASH filed by the Affiliated Hospital of Hangzhou Normal University.
- US2023348983A1: A method for detecting MASH progression using non-invasive techniques filed by Cambridge Enterprise Ltd. and Genome Research Ltd.
- WO2023187713A1: Covers a system utilizing a combination of biomarkers to effectively monitor MASH developed by the Catholic University of Korea.
Kits & PoC AIML:
- Significant Technology Advancement:
- Novel point-of-care testing devices with improved sensitivity and specificity.
- AI-based algorithms for real-time monitoring and prediction of patient health.
- Integration of point-of-care testing with electronic health record systems.
- Wearable devices for continuous monitoring of vital signs and biomarkers.
- Companion diagnostic tools for personalized MASH treatment.
- Key Patents:
- WO2023203083A1: This patent is related to AI-enhanced diagnostic systems assigned to Institut National de la Sante et de la Recherche Medicale.
- US2023407264A1: Focuses on AI algorithms designed for real-time health monitoring filed by Goliver Therapeutics.
- JP2023550278A: Involves innovative biosensor technology developed by Hoffmann La Roche AG.
Key Insights from the Patent Landscape Analysis
- Leaders in Patent Filings: The landscape highlights that Genfit, Roche, and Brexogen Inc. are among the top entities with the most extensive patent portfolios in this area. These companies have focused their efforts on developing diagnostic tools and methods that can offer an early and more accurate diagnosis of liver diseases.
- Strategic Positioning through Patents: The patents cover a variety of innovative methods and technologies, including non-invasive biomarkers, diagnostic blood testing, and AI-enhanced diagnostic processes. The strategic use of patents allows these companies to establish a significant presence in the market and potentially block competitors from entering the space with similar technologies.
- Commercial Impact of Patent Portfolios: The strong patent portfolios of these companies not only protect their R&D investments but also enhance their ability to negotiate partnerships and collaborations. For example, the patent analysis shows how companies leverage their patented technologies to enter clinical trials or to collaborate with other pharmaceutical giants to accelerate product development and market entry.
- Innovative Focus Areas: The landscape analysis shows that recent patents involve the use of advanced technologies like artificial intelligence to improve the accuracy and efficiency of diagnostics. This reflects a broader trend in the healthcare sector, where AI integration into diagnostic processes is becoming a competitive edge.
- Market Dynamics and Future Trends: The patent analysis predicts that continued innovation and patenting activity will drive the future of diagnostics in liver health. It notes that the market is moving towards more personalized and less invasive testing methods, driven by the need for better patient compliance and more accurate diagnostics.
Emerging Companies in MASH Diagnostics: Technology and Clinical Stage
Metadeq:
- Technology: Metadeq focuses on developing advanced biomarker-based diagnostic tools for MASH. Their technology leverages multi-omics approaches, including genomics, proteomics, and metabolomics, to identify novel biomarkers that can be used for early detection and monitoring of MASH progression. Metadeq’s platform integrates these biomarkers into a comprehensive diagnostic panel that offers high sensitivity and specificity.
- Clinical Stage: Metadeq has completed a clinical study with published results in Gut, which showed their predictive algorithm using AI provided unprecedented sensitivity (88-95%), specificity (90-100%), and overall accuracy (92-93%) for MASH. It also has near-perfect sensitivity (99-100%), specificity (90-96%), and accuracy (98-99%) for liver fibrosis. The test results show that the proteins can provide a rapid, cost-effective, and non-invasive testing solution to combat the growing epidemic of MASH and liver fibrosis. This could be an invaluable tool in diagnosing and monitoring cases of liver diseases, allowing people to receive earlier treatment from lifestyle adjustments to surgical and pharmacological interventions.
Endra Life Sciences:
- Technology: Endra Life Sciences has developed the Thermo Acoustic Enhanced Ultrasound (TAEUS) technology, which aims to provide a more accessible and cost-effective alternative to MRI for assessing liver fat, a key indicator of MASH. TAEUS works by measuring tissue composition, including fat and other biomarkers, using sound waves and thermal energy.
- Clinical Stage: Endra is in the clinical validation stage with its TAEUS technology and has submitted for De Novo approval from the FDA based on clinical data from 45 patients. The company has completed multiple pilot studies and is currently conducting pivotal trials to further validate the accuracy and reliability of TAEUS in diagnosing and monitoring MASH. They have received regulatory clearance in some regions and are working towards broader market approval.
Fibronest:
- Technology: Fibronest specializes in developing AI-driven imaging solutions for the non-invasive assessment of liver fibrosis, a critical component of MASH diagnosis. Their technology combines advanced imaging techniques with machine learning algorithms to analyze liver tissue characteristics and quantify fibrosis levels accurately.
- Clinical Stage: Fibronest has completed numerous clinical studies and has entered commercial partnerships. Their AI-driven platform has shown potential in improving diagnostic accuracy and reducing the need for invasive liver biopsies.
Insights for Competitors
Some key insights for competitors in the MASH/MASLD and liver fibrosis diagnostics space are discussed below:
- Invest in research and development to identify novel biomarkers and develop cutting-edge diagnostic technologies.
- Explore the potential of emerging technologies such as AI/ML, multi-omics, and digital health solutions.
- Continuously improve the accuracy, reliability, and user-friendliness of diagnostic products.
- Develop non-invasive and patient-centric diagnostic solutions to address unmet needs.
The insights and analysis presented in this report were derived from IP Research work performed for MetaDeq Diagnostics and ENDRA Life Sciences, active clients of our firm. Our firm also holds an equity interest in both companies and, in the case of ENDRA, shares a common board member. As such, readers should be aware of the potential for a conflict of interest or bias in our analysis. However, we maintain our commitment to providing accurate and objective insights, and our findings are based on thorough research of the IP landscape. We encourage readers to consider this disclosure when interpreting the information presented in this report.
About PatentVest
PatentVest, a division of MDB Capital Holdings (Nasdaq: MDBH), is the first integrated IP intelligence strategy and law firm to enable visionary companies to develop into technology leaders. By combining our proprietary database with a time-proven IP diligence process and expert analysis, we deliver actionable insights on the IP landscape to help our clients make informed decisions and stay ahead of the curve. The trends and competitive insights in this report are powered by PatentVest’s proprietary IP intelligence platform. Our reports keep a pulse on the key players, technologies, and opportunities shaping deep technology markets.
